High-throughput volumetric optical imaging
To rapidly collect the vast amount of biological information from intact systems, a pressing need for large-scale studies, we set our sights on the next leap forward in imaging throughput. Breaking the long-standing light penetration barrier, our methods now open up large biological systems for optical access. To fully harness these massive imaging volumes, we develop approaches that use photons efficiently to boost imaging throughput, such as depth of focus (DOF) extension and full-space scattering potential reconstruction.
The fundamental questions we aim to answer are:
- Does an imaging throughput limit exist for a given amount of photons?
- How does this physical limit link to the amount of unbiased information we can acquire from a system – how many bits can a single photon possibly generate?
Long-lasting nano-scale proteomic contrast synthesis
Aiming to bring the high-throughput imaging to the cellular/molecular level, we develop new biomarkers that scatter coherent photons. This development is critical to enable quantitative biological investigation because scattering reporters, unlike the traditional fluorophors with photo-bleaching limit, exhibit long-lasting contrast that is pivotal for prolonged light exposure in large-scale imaging.
We synthesize scattering nano-constructs to label specific proteins and demonstrated their visualization in large tissue samples with high molecular specificity and at high throughput by combining our labeling and imaging approaches. We enhance tissue processing and labeling with advanced polymer, electrophoresis, and light-activated chemistry techniques.
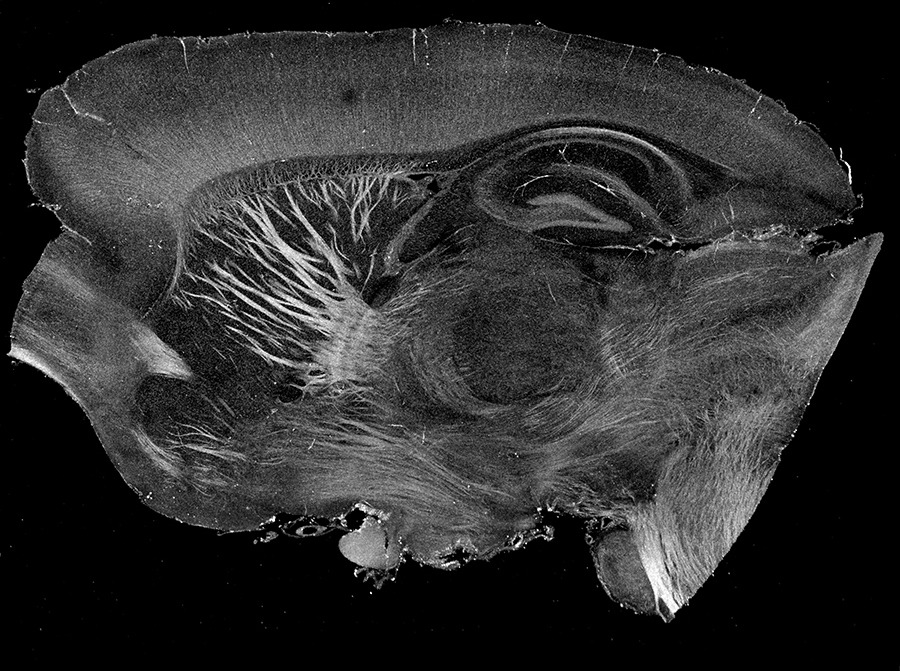
Cross-scale cross-modality image validation, graph analysis, and topological abstraction
We set out to build computational tools for scalable analysis of the newly available ‘big’ data from our imaging methods. Our goal is to uncover the unseen biology buried in large-scale datasets and provide system-wide insights for some long-standing biological questions. Through techniques such as full-space reconstruction, image enhancement, and topological abstract, we offer new perspectives and the most biologically essential information for large-scale investigations.
One example is that we improve algorithm efficiency to reconstruct tractography and build graph models, which disclose the topological connections among brain regions. It not only provides brain wiring diagrams that are rooted in ground-truth anatomy but also enables cross-scale data fusion with MRI. Leveraging cutting-edge parallel computing and machine learning, we are passionate about further advancing the computational techniques capable of processing our data.
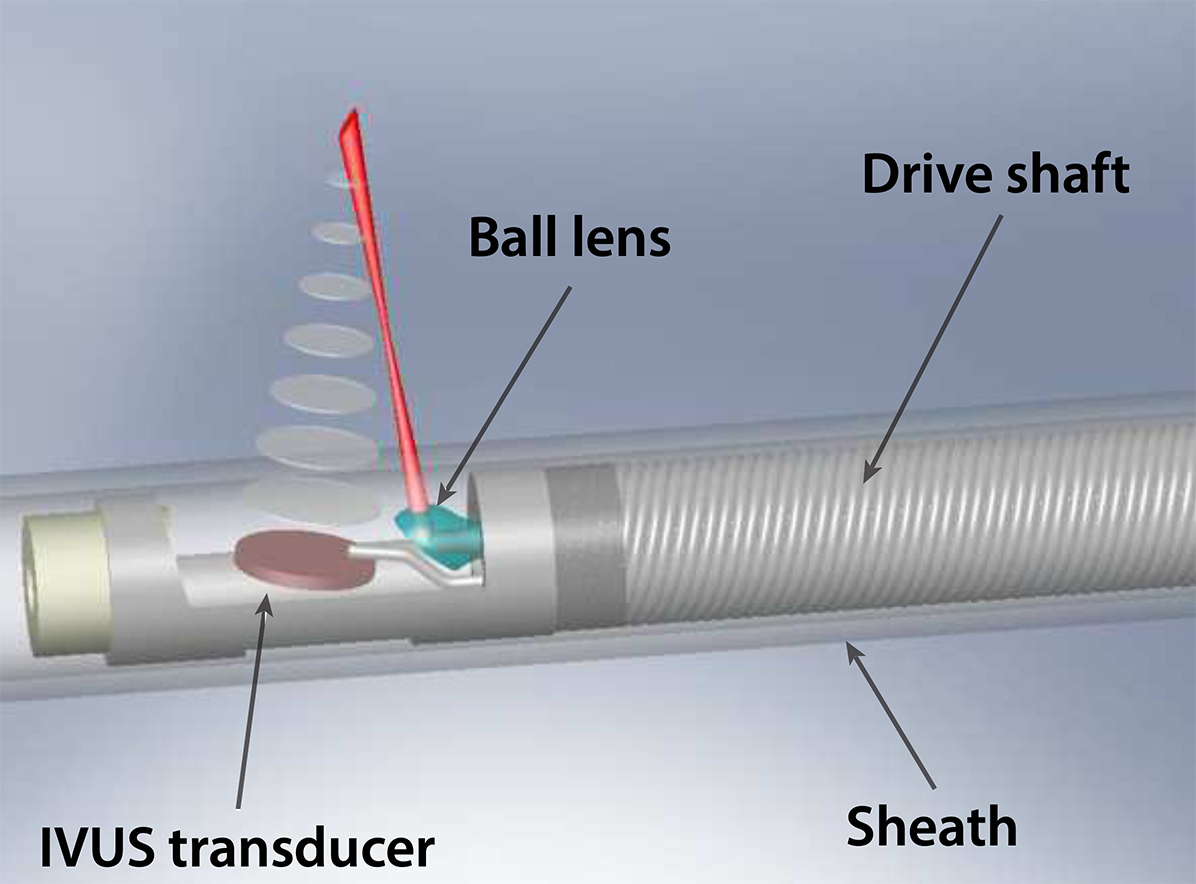
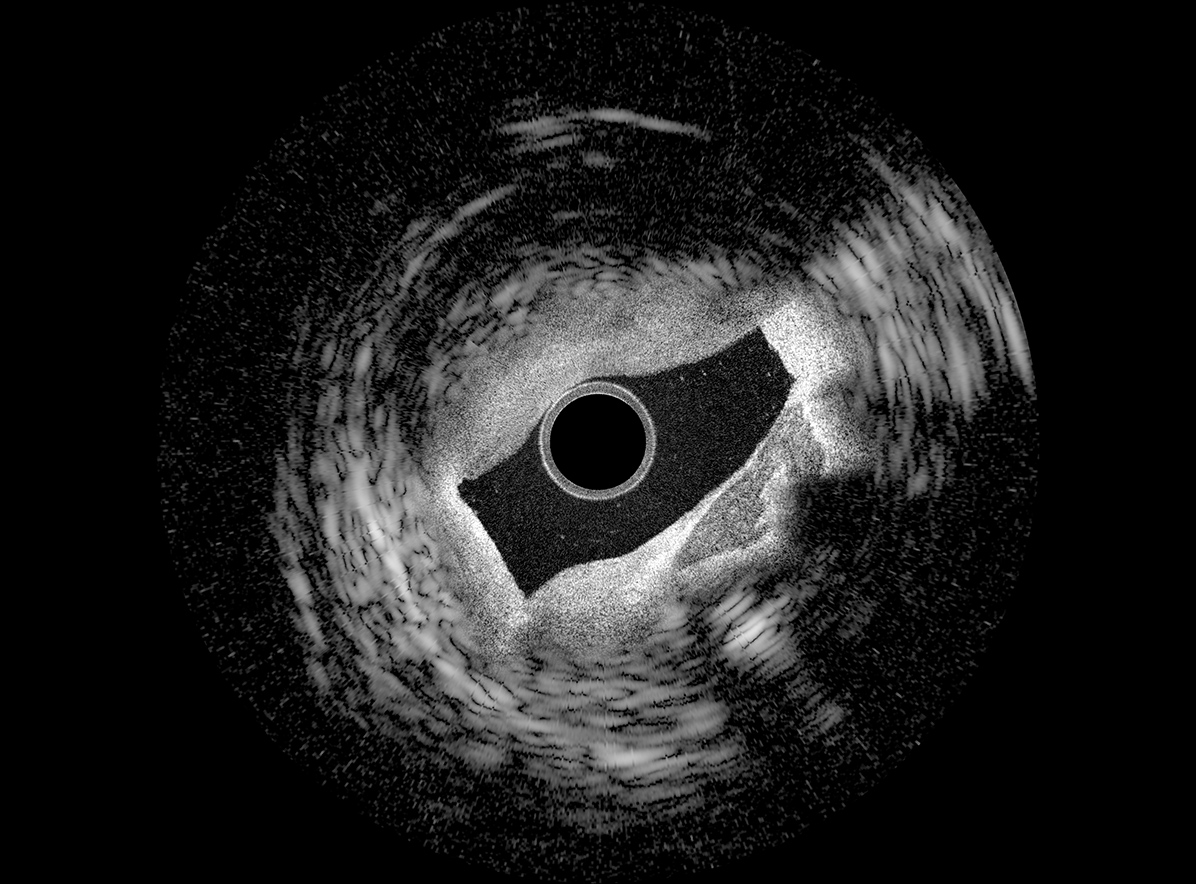
Translational tools: multimodal systems and miniaturized devices
We are enthusiastic about developing enabling medical tools empowered by new photonic devices and integrating complementary modalities for a wide range of clinical applications. We build various prototype and preclinical imaging probes and systems, which are now opening new opportunities for endoscopic procedures. Exploiting techniques from computer vision, optical and mechanical engineering, nanofabrication, and nonlinear optics, our devices have shown promise in providing intraoperative guidance for ophthalmology, orthopedics, and interventional cardiology.
Building from this expertise, we are keen to work closely with our physician partners to understand their unmet clinical needs and seek innovative solutions enabled by next-generation miniaturized devices and multimodal systems.